Background
Targeting the PI3K/Akt/mTOR axis in relapsed lymphomas is of interest based on constitutive activation in many lymphoma subtypes, and has had varying degrees of success. We previously showed that the first generation mTOR inhibitor, temsirolimus (TEM), has activity across histologies with an acceptable toxicity profile (Smith, et al., JCO 2010). Lenalidomide (LEN) is currently approved for use in indolent non-Hodgkin lymphomas, and has several potential synergistic and overlapping targets with PI3K/mTOR/Akt inhibition. We designed this phase I/II clinical trial to evaluate the efficacy and tolerability of the combination of TEM and LEN in relapsed/refractory lymphomas.
Methods
The phase I dose-finding study utilized a standard "3+3" design and was open to all patients with mature B-cell malignancies. TEM was 25 mg IV weekly for all dose levels. LEN was dosed orally on D1-D21 every 28 days at three dose levels: 15 mg, 20 mg, and 25 mg. The phase II study accrued patients in a two-stage "minimax" design with stratification into three histologically-defined cohorts: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and other lymphomas. Primary endpoints of the phase II study were rates of complete (CR) and overall response (ORR), and secondary endpoints were duration of response (DOR), progression-free survival (PFS) and overall survival (OS).
Results
In the phase I study, 18 patients were enrolled and available for toxicity assessment. Patients were treated to intolerance, progression, or discontinuation at physician discretion. Of these, 15 patients were evaluable for dose-limiting toxicity (DLT) assessment. At dose level 3, there were 2 DLTs: grade 3 diarrhea and grade 3 HSV mucositis. Dose level 2 was thus established as the recommended phase II dose: TEM 25 mg weekly and LEN 20 mg on D1-D21 every 28 days. Of the 18 patients, there were 5 partial responses, 4 stable disease, 3 progressive disease, 4 on active treatment, and 2 not adequately assessed.
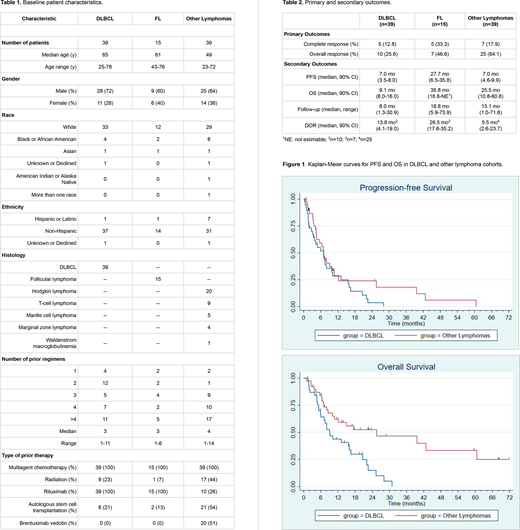
The phase II study enrolled an additional 93 patients (Table 1): 39 DLBCL, 15 FL, and 39 other lymphomas which included 20 relapsed/refractory Hodgkin lymphoma (HL) patients. The median number of prior treatments was 4 (range, 1-14), and 31 patients (33%) had relapsed following prior autologous stem cell transplantation (ASCT). The median number of cycles delivered was 4 (range, 1-21). The FL cohort closed prematurely due to slow accrual.
The ORR were 25.6% (12.8% CR) and 64.1% (17.9% CR) for DLBCL and other lymphoma cohorts, respectively (Table 2). The ORR for HL patients in the other lymphoma cohort, the majority of whom had relapsed after brentuximab vedotin (BV) and autologous stem cell transplantation (ASCT), was 80% (35% CR). Eight HL patients (40%) proceeded to allogeneic transplantation after TEM and LEN therapy. The high response rate in the other lymphoma cohort was sufficient to reject the null hypothesis of a 30% response rate under the minimax design.
Median PFS was 7.0 mo (90% CI 3.5-8.0) and 7.0 mo (90% CI 4.6-9.9) for DLBCL and other lymphoma cohorts, respectively (Table 2). Median OS was 9.1 mo (90% CI 6.0-16.0) and 25.5 mo (90% CI 10.8-60.6) for DLBCL and other lymphoma cohorts, respectively (Table 2). Median DOR was 13.8 mo (90% CI 4.1-19.0) and 5.5 mo (90% CI 2.6-23.7) for DLBCL and other lymphoma cohorts, respectively (Table 2). Median PFS, OS and DOR for HL patients in the other lymphoma cohort were 9.2 mo (90% CI 4.6-25.5), 39.6 mo (90% CI 17.4-NR), and 8.1 mo (90% CI 5.1-38.3), respectively. Kaplan-Meier curves are displayed in Figure 1.
Grade ≥3 non-hematologic adverse events (AE) related to treatment were uncommon, with no cases of pneumonitis and one grade 3 thromboembolism. Grade ≥3 hematologic AEs were common and reversible. Three Grade 5 AEs occurred (colonic perforation, myocardial infarction and sepsis).
Conclusions
Combination therapy with TEM and LEN demonstrated encouraging activity in heavily-pretreated and relapsed/refractory lymphomas. Survival in the other lymphoma cohort was primarily driven by favorable activity in relapsed/refractory HL. TEM and LEN may be a suitable option for treatment of HL after BV and ASCT, including as a bridge to allogeneic stem cell transplantation. Further study of PI3K/Akt/mTOR inhibition in combination with lenalidomide is warranted, particularly in relapsed HL.
Kline:Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Kite/Gilead: Speakers Bureau. Kimball:Amgen: Current Employment; Amgen: Current equity holder in publicly-traded company. Petrich:Daiichi-Sankyo: Current Employment; AbbVie: Current equity holder in publicly-traded company. Smith:Celgene: Consultancy, Research Funding; Janssen: Consultancy; Genentech/Roche: Consultancy, Other: Support of parent study and funding of editorial support, Research Funding; TG Therapeutics: Consultancy, Research Funding; FortySeven: Research Funding; Pharmacyclics: Research Funding; Karyopharm: Consultancy, Research Funding; BMS: Consultancy; Acerta: Research Funding.
Temsirolimus is FDA-approved for renal cell carcinoma.
Author notes
Asterisk with author names denotes non-ASH members.